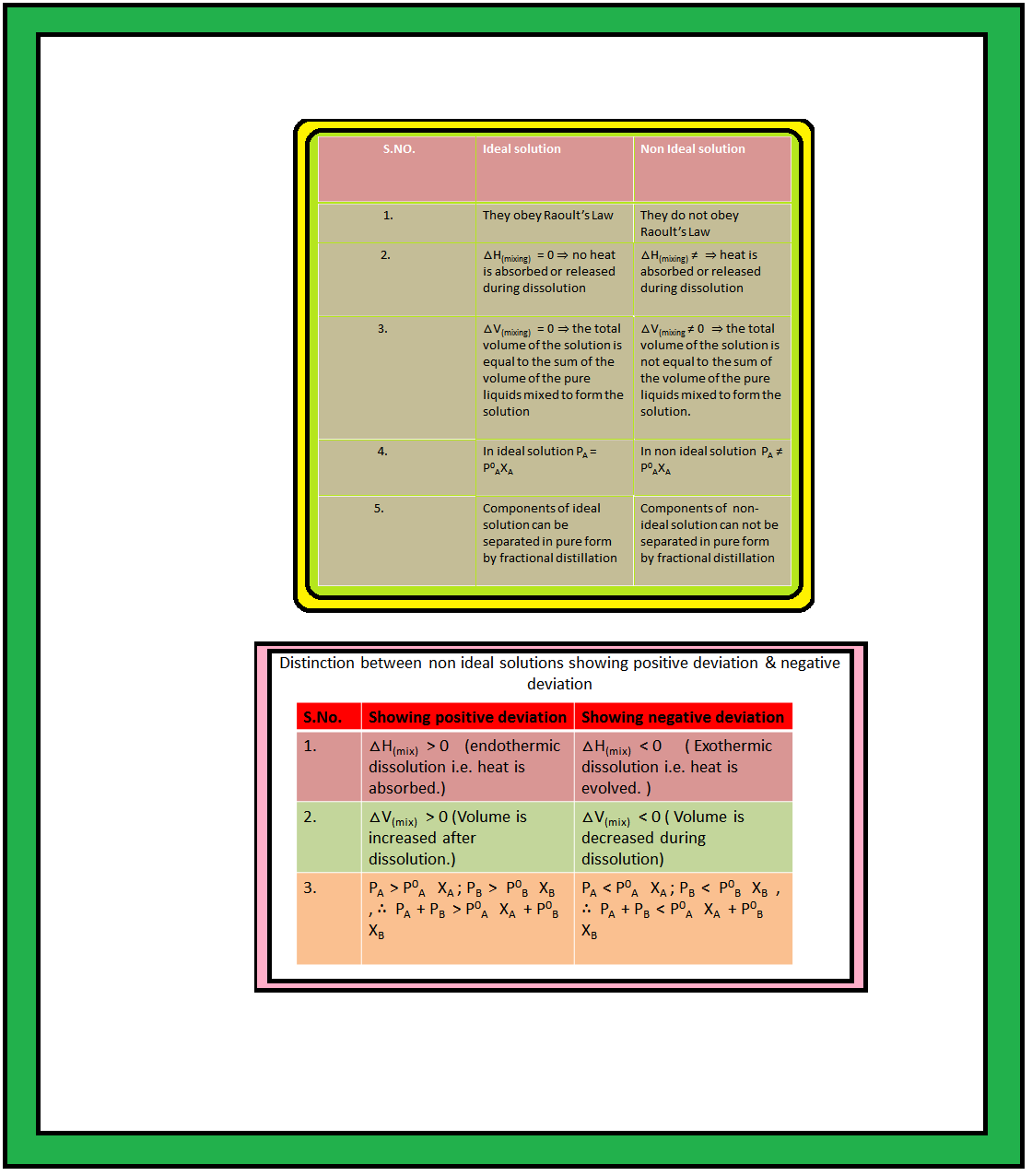
`color{green}("Definition") :` When a solution does not obey Raoult’s law over the entire range of concentration, then it is called non-ideal solution.
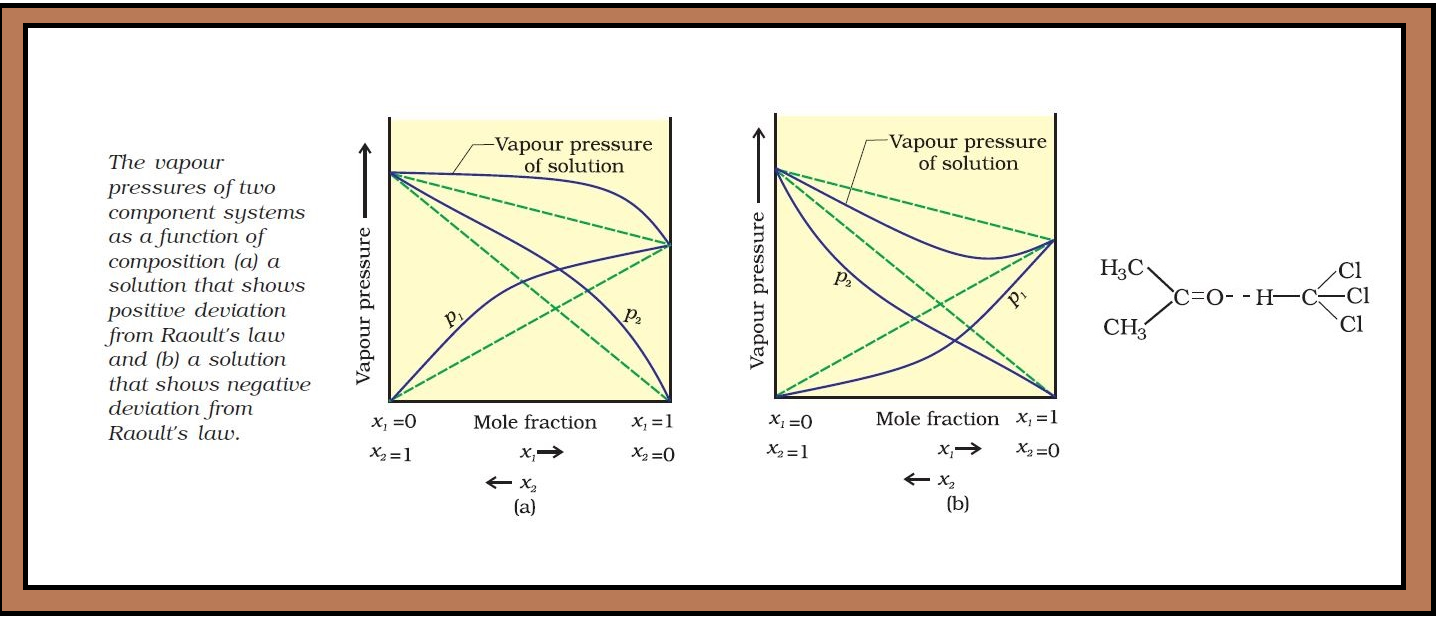
`=>` The vapour pressure of such a solution is either higher or lower than that predicted by Raoult’s law (equation 2.16). The plots of vapour pressure as a function of mole fractions for such solutions are shown in Fig. 2.6.
`color{green}("Positive Deviation") :` If vapour pressure of a solution is higher than that predicted by Raoult’s law, the solution exhibits positive deviation.
`->` In case of positive deviation from Raoult’s law, `A-B` interactions are weaker than those between `A-A` or `B-B`, i.e., in this case the intermolecular attractive forces between the solute-solvent molecules are weaker than those between the solute-solute and solvent-solvent molecules.
`->` In such solutions, molecules of `A` (or `B`) will find it easier to escape than in pure state. This will increase the vapour pressure and result in positive deviation.
`color{red}("Examples") :` (i) Mixtures of ethanol and acetone behave in this manner. In pure ethanol, molecules are hydrogen bonded. On adding acetone, its molecules get in between the ethanol molecules and break some of the hydrogen bonds between them. Due to weakening of interactions, the solution shows positive deviation from Raoult’s law [Fig. 2.6 (a)].
(ii) A solution formed by adding carbon disulphide to acetone, the dipolar interactions between solute-solvent molecules are weaker than the respective interactions among the solute-solute and solvent-solvent molecules. This solution also shows positive deviation.
`color{green}("Negative Deviation") :` If vapour pressure of a solution is lower than that predicted by Raoult’s law, it exhibits negative deviation.
`->` In case of negative deviations from Raoult’s law, the intermolecular attractive forces between `A-A` and `B-B` are weaker than those between `A-B` and leads to decrease in vapour pressure resulting in negative deviations.
`color{red}("Examples") :` (i) Mixture of phenol and aniline. In this case, the intermolecular hydrogen bonding between phenolic proton and lone pair on nitrogen atom of aniline is stronger than the respective intermolecular hydrogen bonding between similar molecules.
(ii) A mixture of chloroform and acetone forms a solution with negative deviation from Raoult’s law. This is because chloroform molecule is able to form hydrogen bond with acetone molecule as shown. This decreases the escaping tendency of molecules for each component and as a result the vapour pressure decreases resulting in negative deviation from Raoult’s law [Fig. 2.6. (b)].
`color{green}("Definition") :` When a solution does not obey Raoult’s law over the entire range of concentration, then it is called non-ideal solution.
`=>` The vapour pressure of such a solution is either higher or lower than that predicted by Raoult’s law (equation 2.16). The plots of vapour pressure as a function of mole fractions for such solutions are shown in Fig. 2.6.
`color{green}("Positive Deviation") :` If vapour pressure of a solution is higher than that predicted by Raoult’s law, the solution exhibits positive deviation.
`->` In case of positive deviation from Raoult’s law, `A-B` interactions are weaker than those between `A-A` or `B-B`, i.e., in this case the intermolecular attractive forces between the solute-solvent molecules are weaker than those between the solute-solute and solvent-solvent molecules.
`->` In such solutions, molecules of `A` (or `B`) will find it easier to escape than in pure state. This will increase the vapour pressure and result in positive deviation.
`color{red}("Examples") :` (i) Mixtures of ethanol and acetone behave in this manner. In pure ethanol, molecules are hydrogen bonded. On adding acetone, its molecules get in between the ethanol molecules and break some of the hydrogen bonds between them. Due to weakening of interactions, the solution shows positive deviation from Raoult’s law [Fig. 2.6 (a)].
(ii) A solution formed by adding carbon disulphide to acetone, the dipolar interactions between solute-solvent molecules are weaker than the respective interactions among the solute-solute and solvent-solvent molecules. This solution also shows positive deviation.
`color{green}("Negative Deviation") :` If vapour pressure of a solution is lower than that predicted by Raoult’s law, it exhibits negative deviation.
`->` In case of negative deviations from Raoult’s law, the intermolecular attractive forces between `A-A` and `B-B` are weaker than those between `A-B` and leads to decrease in vapour pressure resulting in negative deviations.
`color{red}("Examples") :` (i) Mixture of phenol and aniline. In this case, the intermolecular hydrogen bonding between phenolic proton and lone pair on nitrogen atom of aniline is stronger than the respective intermolecular hydrogen bonding between similar molecules.
(ii) A mixture of chloroform and acetone forms a solution with negative deviation from Raoult’s law. This is because chloroform molecule is able to form hydrogen bond with acetone molecule as shown. This decreases the escaping tendency of molecules for each component and as a result the vapour pressure decreases resulting in negative deviation from Raoult’s law [Fig. 2.6. (b)].